Abstract
Introduction:
Despite a plethora of novel therapies, autologous stem cell transplant (ASCT) continues to offer a progression free survival benefit to multiple myeloma patients. At our institution, standard of care for transplant-eligible patients remains induction with cyclophosphamide, bortezomib, and decadron (CyBorD) for 4 cycles, followed by ASCT. However, limited resources for collection and the logistics of receiving late referrals from outside centers often result in patients receiving additional cycles of treatment prior to transplant. We sought to determine whether the administration of additional induction chemo led to increased peri-transplant morbidity.
Methods:
With REB approval, chart review was done for myeloma patients receiving an ASCT between 2007 and 2017. Only patients receiving induction with CyBorD or bortezomib-decadron on a like schedule were included in analysis. Patients received pre-ASCT conditioning of high-dose melphalan (93.5%) or busulfan-melphalan (6.5%). Data collected included patient's age, gender, myeloma subtype, ISS score, and cytogenetics, as well as the number of induction cycles received and hematopoietic cell transplantation-specific co-morbidity index (HCT-CI). The primary endpoint assessed was median length of hospital stay (LOS) post-infusion. Secondary end-points included time to engraftment, ICU transfer rate, infection rate, organ-specific toxicity, 100-day mortality, and response as measured by VGPR rate at 100 days. Patients were grouped into those who received 2-4 cycles vs. 5-9 cycles of induction.
Results:
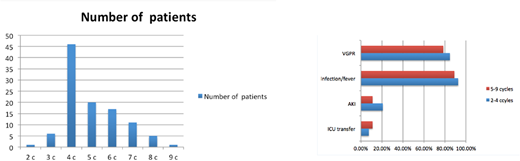
Fifty-three patients received 2-4 cycles of induction and 54 received 5-9. Median age was 61 for both groups, gender was well-matched (53% vs. 57% male), and HCT-CI ≥ 3 was similar (26% vs. 31%).The only significant difference between groups was the subtype of myeloma, with the 2-4 group having 30.1% light chain disease (vs. 14.8%) and 39.6% IgG subtype (vs. 61.1%) (p = 0.026). Median LOS post-infusion was 20 days in the 2-4 group and 17 days in the 5-9 group. Median time to engraftment was 12 days in both (range 9-21 in 2-4 group, 10-20 in 5-9 group). ICU transfer was needed in 4 (7.5%) and 6 (11.1%) patients in the respective groups, and there were two deaths in the 5-9 group (one of sepsis at d+16 and one of multi-organ failure at d+46). Both patients had HCT-CI scores of 5, and the differences in ICU and mortality rates were not statistically significant. There were no significant differences in measurable toxicity. Documented infection or febrile neutropenia occurred in 92.5% vs. 88.9% (p = 0.53); acute kidney injury in 20.8% vs. 11.1% (p= 0.17); and acute liver injury in 24.5% vs. 14.8% (p = 0.21). Among evaluable patients, 84.6% achieved VGPR or better in the 2-4 group and 78.4% in the 5-9 group, although the latter had a higher number of patients with high-risk cytogenetics by FISH (6 vs. 10 with t(4;14) or 17p-).
Discussion:
Evidence for a precise number of cycles of induction prior to ASCT is scarce. A recent study by Charaborty et. al.(BJHJul 2018) showed that median PFS and OS were similar between patients who received ≤ 4 months vs. > 4 months of induction. 39% of their patients received bortezomib-based induction without lenalidomide, akin to our regimen. Likewise, our study showed no significant difference in VGPR rates at 100 days, an endpoint used at our center as one criterion for determining the need for tandem transplant and potential increased chemotherapy exposure for the patient. Our overall VGPR rate for the group of 81.6% is comparable to previously published results, suggesting our population is representative of a typical myeloma cohort. Most importantly, our data suggests no increase in peri-transplant toxicity as a result of the additional induction chemotherapy burden. In fact, there was a surprising trend towards decreased organ toxicity and shorter hospital stay, and the only two early deaths occurred in high-risk patients. Among the 17 patients who received the heaviest induction burden (7-9 cycles), there were no deaths and only 1 ICU transfer. While there did not appear to be any long-term benefit to greater induction length, our study provides reassurance that it is safe to continue CyBorD induction past the intended 4 cycles without compromising patient safety at the time of transplant.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal